The role of liquid biopsy in precision medicine
In the third part of our blog series, we will discuss how liquid biopsy have revolutionized the field of precision medicine and opened up new ways in cancer management.
Precision medicine in oncology aims to understand the molecular mechanisms behind cancer development based on unique characteristics, individual genomic aberrations in patients’ tumor in order to improve therapeutic response and reduce treatment side effects. Through identifying driver oncogenic pathways with a set of genetic and protein expression assays oncologists can select the most beneficial targeted anti-cancer therapy for individual patients (Dudek et al., 2020). Unlike the traditional paradigm of treating classes of patients based on the cancer’s tissue type, the objective shifted to achieve tailored treatments for each individual patient, in real time, as their tumor evolves during treatment (Le Tourneau et al, 2016).
Tumors are dependent on oncogenes and tumor-suppressor genes. Modifications of these genes lead to the appearance of tumor cells. The activation process leading to so-called proto-oncogenes are chromosomal translocation, point mutation, and gene amplification. Proto-oncogenes are the first regulatory factors in the signaling pathways for cells to grow and survive, therefore they are in close association with tumor development and inhibition of apoptosis or programmed cell death, providing cell immortality. Tumor-suppressor genes on the contrary would block the growth of cancer and contribute to the normal development of cells but inherited or acquired mutations in these genes may also contribute to tumor development (Kontomanolis et al., 2020). By understanding the molecular mechanisms of tumor development new drug targets can be identified and by detecting the genetic changes that occur during therapy, new effective strategies can be used in the treatment of cancer patients (Tsimberidou et al., 2015).
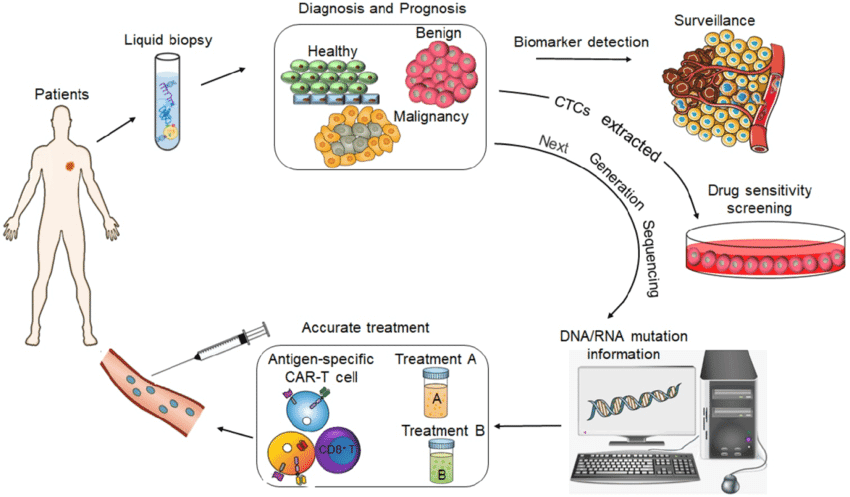
Liquid biopsies consisting of isolating tumor-derived entities – circulating tumor cells (CTCs), circulating tumor DNA (ctDNA), etc. – present in the body fluids of patients with cancer offers continuous monitoring by repeated sampling. The downstream genomic and proteomic analysis of liquid biopsy mediums can identify biomarkers for personalized therapeutic regimens, and screening for therapeutic resistance in a dynamic way (Fig. 1).
Fig.1. Schematic figure of liquid biopsy analysis in precision medicine (Wu et al.,2020).
Liquid biopsy has not replaced tumor tissue biopsies as far as cancer diagnosis is concerned, but it adds unsurpassed benefits to cancer management. Besides the detection of genetic alterations at the time of diagnosis liquid biopsy also allows the tracking of the so-called „clonal evolution” of the tumor in real-time. There is growing evidence that some key genes can mutate over time in tumors because of „selective” pressure exerted by therapy or because of tumor microenvironment, generating alterations responsible for resistance to cancer therapies. Liquid biopsy-based methods can detect the changes in the tumor, providing an opportunity to refine therapeutic strategies according to the new molecular properties of the tumor.
A characteristic of advanced tumors is the high heterogeneity. For example, metastases that spread from a primary cancer to other areas of the body can have distinct types of abnormalities in an individual patient. Performing a liquid biopsy, on the other hand, allows to receive tumor DNA from multiple locations and thus have a broader and heterogeneous picture.
In recent years, the Food and Drug Administration (FDA) has approved some liquid biopsy tests called „companion diagnostics” (CDx), to match certain driver mutations to potentially effective target treatments.
The first liquid biopsy based CDx was approved in lung cancer. Non-small cell lung cancer (NSCL) often presents at least one driver mutation. The main changes identified were in the epidermal growth factor receptor (EGFR) and in the anaplastic lymphoma kinase (ALK), both protein tyrosine kinases receptors, responsible for gene expression, acting in cell growth, survival, migration, and apoptosis, these being, the main targets for the treatment of NSCLC. The discovery of these molecular alterations changed the course of the treatment for patients with NSCLC, as it enabled the development of tyrosine kinase inhibitors (TKIs). The response to the use of TKIs has been promising, with significant clinical benefits. Objective response rates of 60–70% are reported with the use of these different TKIs and a disease control rate of up to 80–90%. However, patients tend to develop drug resistance within 1 to 2 years due to somatic mutations in the EGFR, but with continuous monitoring of the disease immediate action can be taken and a change in therapy can lead to better outcome (Torres, 2021).
Studies have shown that CTCs have predictive, diagnostic, and prognostic value to identify driver mutations, in addition to identifying and monitoring mutations related to resistance to treatments in several types of cancer, including NSCLC (Pawlikowska et al., 2019, Kloten et al., 2019, O’Flaherty et al., 2017), breast cancer (Banuelos et al., 2020, Schochter et al., 2019), colorectal cancer (Yamada et al., 2019), prostate cancer (Pantel et al., 2019), etc.
In clinical practice, liquid biopsy has often been used to evaluate patients with advanced cancer, to determine if they could be candidates for targeted treatments or to better understand their prognosis. Liquid biopsy offers the potential to see the genetic diversity within a tumor, also enabling longitudinal measurements to monitor genetic changes over time. In fact, sometimes a liquid biopsy may be the only way to obtain genetic information, when the tumor site is inaccessible or if the pathological sample is inadequate. The acquired information with liquid biopsy will make it possible to design new protocols for less invasive oncological surveillance, drastically improve personalized treatment strategies and advance the field of precision medicine.
Recent blogs
Cell-free DNA Fragmentomics: A Promising Predictor of Cancer
In this blog entry, we will explore the recent history, intriguing findings, and tools related to cfDNA fragmentomics.
New developments in the field of circulating tumor cells (2024)
The blog post focuses on how researchers can produce more meaningful, applicable results that directly benefit human health.
Singlera technologies 5: Panseer – detecting pan-cancer signatures years before conventional diagnosis
In the last blog of the year and the concluding chapter of the Singlera series, we are going to explore PanSeer, a blood-based screening test utilizing unique methylation signatures.