Refining Cancer Treatment Monitoring Using Liquid Biopsy
Liquid biopsy has broad application possibilities in cancer diagnosis and patient monitoring, covering involvement in early diagnosis, prognosis as well as monitoring of therapeutic response. In the second part of our blog series, we will explore the topic of drug response assessment in cancer treatment with liquid biopsy-based methods.
Over the last decade new genetically targeted cancer drugs, immunotherapies – in particular, immune checkpoint inhibitors (ICI) – revolutionized cancer treatment, providing better prospects than traditional chemotherapy. At the same time transition into clinical oncology has been slow due to the high cost of treatment, associated toxicity and variability of the response between patients. In response, personalized approaches using predictive biomarkers have emerged as new tools for patient stratification and to achieve effective therapy.
Recently, the enumeration and molecular analysis of circulating tumor cells (CTCs) have been highlighted as prognostic and predictive biomarker for the management of cancer patients during chemotherapy, hormonal therapy and for targeted therapy in a personalized manner (Rzhevskiy et al., 2021). In contrast to tissue sampling, monitoring the successfulness or failure of systemic therapies by sequential measurements of CTCs is more feasible, and it is not limited by sampling frequency or tumor accessibility.
One of the main advantages of using CTCs as a biomarker in cancer treatment and resistance monitoring is that they can provide quantitative information on treatment response by enumerating CTCs and allow qualitative clarification of resistance mechanisms through tumor genotyping, but they can be also used in vitro to test drug therapy before the actual administration of therapy and consequently identify potential drug resistances or drug sensitivity (Labib et al., 2021, Smit et al., 2021).
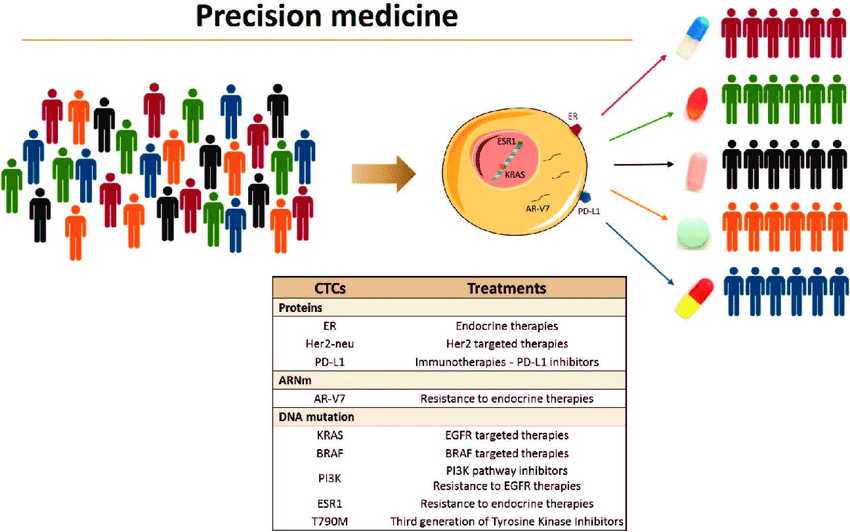
CTC molecular profiling supports personalized cancer treatment aiming to tailor the most effective therapy options to each individual cancer patient based on the molecular profile of the tumor of each individual patient (Hirahata et al., 2022). Real-time monitoring of therapeutic efficacy, and detection of therapeutic targets and resistance mechanisms are becoming also increasingly important in oncology (Alix-Panabières and Pantel, 2021).
Markers of drug efficiency
Although the enumeration of CTCs has been associated with progression free survival and overall survival across several solid tumors (breast (Cristofanilli et al., 2004), lung (Punnoose et al, 2012), prostate (Panteleakou et al., 2019), colorectal (Cohen et al., 2008) tumors, etc.) several studies also indicate that CTCs can be used as a surrogate marker of response to therapy, especially considering PD-L1 inhibitors, which are emerging ICI regimes with less toxicity and long-term effects for a number of tumors (Philips et al, 2014).
Blocking immune checkpoints has been one of the focuses of antitumor immunotherapy. By blocking the immune checkpoint on CTCs, the immune system can be activated to eliminate CTCs from the blood, which suggests a new way to reduce the recurrence and metastasis of tumors (Zhong et al., 2020).
Recent reports have shown frequent expression of immune checkpoint proteins on CTCs such as PD-L1, which can be assessed prior to and during therapy to understand dynamic tumor genesis in real time. It was also confirmed that the tumor mutation load in plasma is correlated with the efficacy of immunological checkpoint inhibitors (Gandara et al., 2018). Several clinical trials with PD-L1 antibodies have been conducted to evaluate clinical applications of CTCs as a potential biomarker in treatment efficacy measurement in various cancers (Rzhevskiy et al., 2021).
Mazel et al. have demonstrated that the expression of PD-L1 highly increased on CTCs obtained from patients with hormone receptor-positive, HER2-negative breast cancer. CTC/PD-L1 analysis might be applied to patients with immune checkpoint blockade as immunoscores because PD-L1 expression categorizes different subsets of CTCs (David, 2015).
CTCs could be an independent indicator for guiding clinical treatment. Scher et al. have used CTCs for therapy response evaluation in patients undergoing castration-resistant prostate cancer. Results proved that CTC enumerations can be used for real-time therapy evaluation.
Further trials are ongoing to validate the findings.
Markers of drug resistance
The emergence of clinical resistance to a previously effective anti-tumor therapy results from gaining molecular alterations in genes or pathways that guide the mechanisms of action of the newly ineffective therapy. CTCs can carry these acquired alterations, sometimes different from those found at the primary sites, therefore can be used to monitor tumor drug resistance by genotyping CTCs.
One of the first CTC-sequencing studies performed EGFR mutational analysis and detected in serial analysis the emergence of mutations (T790M) in this gene conferring resistance against tyrosine kinase inhibitors in patients with non-small cell lung cancer (Maheswaran et al., 2008). Direct monitoring of T790M mutation in CTCs was employed to potentially guide treatment selection on EGFR-mutant non-small cell lung cancer (NSCLC) patients (Sundaresan et al., 2016). Whole-genome sequencing of single CTCs isolated from NSCLC patients revealed mutational signatures that were shared with metastases that were found 10 months after CTC analyses. Remarkably, these signatures were not detectable in matched primary tumors, suggesting the potential utility of CTC genomic signatures in inferring metastatic propensity and disease relapse (Chemi et al., 2019).
Genetic analyses of CTCs aids targeted therapy investigations. It could be a minimally invasive approach to identify drug sensitivity or resistance-associated markers to guide therapeutic decisions.
In colorectal cancer certain KRAS mutations are established as a negative predictive marker for treatment with EGFR inhibitors. Several studies have successfully detected mutations in KRAS and PI3KCA from CTCs isolated from patients with colorectal cancer. Deep sequencing analysis revealed that mutations in known driver genes (APC, KRAS, and PIK3CA) found in the primary tumor and metastasis were detected in corresponding CTCs. Moreover, other mutations that were initially found only in CTCs were also present at a subclonal level in the primary tumor and metastasis (Tan et al., 2016).
Fig.1. CTCs as liquid biopsy for precision medicine (Cayrefourcq and Alix-Panabières, 2019).
Collectively, these works nicely show heterogeneity of CTC which has enormous implications for precision medicine, including treatment monitoring and treatment resistance assessment.
Evaluating new therapeutic strategies
CTCs collected from distinct treatment stages may reveal the evolving tumor characteristics and potentially capture the dynamic evolution of the tumor, which may provide important clues in devising more effective cancer therapies. Enriched CTCs can therefore be further used to establish primary cell cultures or permanent cell lines or be expanded in vivo using xenografts. Such studies allow the malignant potential of CTCs to be determined and the efficiency of therapeutic agents to be tested. In future, this approach may determine the choice of therapeutic regimen that would be beneficial for a given patient from whom CTCs were sampled before therapy (Kowalik et al, 2017).
Ultimate therapeutic success will require the integration of real-time diagnostic measurements and targeted interventions, which can be achieved by systematic monitoring of CTCs. Hopefully, we have been able to provide a glimpse into the potential of CTCs as a valuable predictive biomarker in cancer management, helping to monitor the efficacy of therapies and detect early development of metastases via their downstream molecular analysis.
Recent blogs
Cell-free DNA Fragmentomics: A Promising Predictor of Cancer
In this blog entry, we will explore the recent history, intriguing findings, and tools related to cfDNA fragmentomics.
New developments in the field of circulating tumor cells (2024)
The blog post focuses on how researchers can produce more meaningful, applicable results that directly benefit human health.
Singlera technologies 5: Panseer – detecting pan-cancer signatures years before conventional diagnosis
In the last blog of the year and the concluding chapter of the Singlera series, we are going to explore PanSeer, a blood-based screening test utilizing unique methylation signatures.